Surface-acoustic-wave biosensors and microfluidics
Surface acoustic waves (SAWs) are acoustic waves that travel along the surface of an elastic material, with an amplitude that typically decays exponentially with depth into the substrate. Given their very superficial nature, SAWs are highly sensitive to surface perturbations of the substrate along which they propagate. For example, they can interact with liquid droplets or streams inducing macroscopic fluid manipulations or, in a different configuration, be exploited for sensing applications. The interest of the NeuroSens group in this field is to explore and study novel SAW-driven microfluidic phenomena, and develop SAW-architectures for sensing applications.
Surface-Acoustic-Wave (SAW) Induced Mixing Enhances the Detection of Viruses: Application to Measles Sensing in Whole Human Saliva with a SAW Lab-On-a-Chip
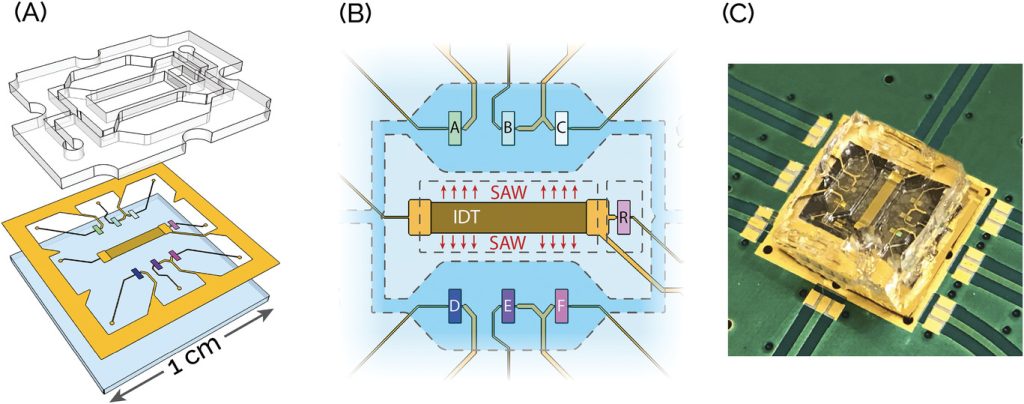
Measles is one of the most infectious airborne viruses worldwide. With a basic reproduction rate between 12–18, this virus is six times more infectious than the SARS-CoV-2 Alpha variant and similar to the SARS-CoV-2 Omicron variant. Even though a cheap and effective vaccine is available, measles is still common in developing countries. To date, sporadic outbreaks are also reported in developed countries, primarily due to non-vaccinated people. This work presents a point-of-care (POC) biosensing device capable of detecting measles virions (MV) in human saliva. The device is a surface-acoustic-wave (SAW) based lab-on-a-chip (LOC), smaller than a €1-cent coin, in which SAWs are used both for sensing and fluid recirculation. The biosensing detection performance of this system is tested and device sensitivity and selectivity are assessed. The SAW-LOC with MV loaded in healthy, whole human saliva is finally validated. The experimental results also highlight a crucial aspect of the biosensing process: the interactions between probing and target species during incubation with or without fluid mixing. The presented findings are promising for realizing a POC platform for measles diagnosis and may serve as a guideline for designing new microfluidics-based biosensing systems.
Glial-fibrillary-acidic-protein (GFAP) biomarker detection in serum-matrix: Functionalization strategies and detection by an ultra-high-frequency surface-acoustic-wave (UHF-SAW) lab-on-chip
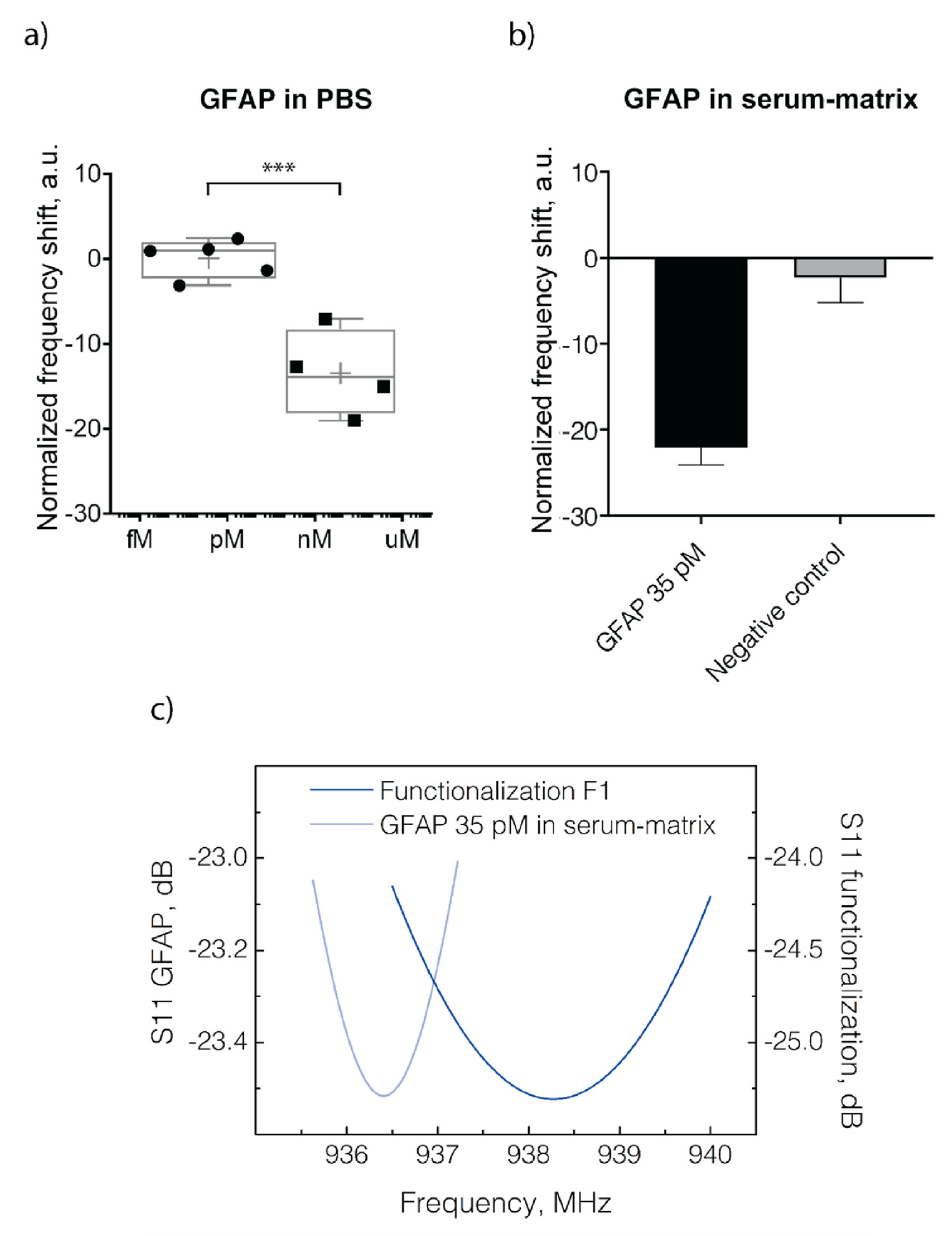
Glial-fibrillary-acidic-protein (GFAP) has recently drawn significant attention from the clinical environment as a promising biomarker. The pathologies which can be linked to the presence of GFAP in blood severely affect the human central nervous system. These pathologies are glioblastoma multiforme (GBM), traumatic brain injuries (TBIs), multiple sclerosis (MS), intracerebral hemorrhage (ICH), and neuromyelitis optica (NMO). Here, we develop three different detection strategies for GFAP, among the most popular in the biosensing field and never examined side by side within the experimental frame. We compare their capability of detecting GFAP in a clean-buffer and serum-matrix by using gold-coated quartz-crystal-microbalance (QCM) sensors. All the three detection strategies are based on antibodies, and each of them focuses on a key aspect of the biosensing process. The first is based on a polyethylene glycol (PEG) chain for antifouling, the second on a protein-G linker for controlling antibody-orientation, and the third on antibody-splitting and direct surface immobilization for high-surface coverage. Then, we select the best-performing protocol and validate its detection performance with an ultra-high-frequency (UHF) surface-acoustic-wave (SAW) based lab-on-chip (LoC). GFAP successful detection is demonstrated in a clean-buffer and serum-matrix at a concentration of 35 pM. This GFAP level is compatible with clinical diagnostics. This result suggests the use of our technology for the realization of a point-of-care biosensing platform for the detection of multiple brain-pathology biomarkers.
Nanotechnologies for the nervous system
The nervous system (NS) is in some ways the most complex and fascinating organ of the human body. Unfortunately, NS pathologies that lead to tissue loss are dramatically difficult to treat because of the negligible regenerative potential of the central nervous system (CNS) from one side, and of the very slow and ineffective repair mechanisms of peripheral nervous system (PNS) from the other side. The interest of the NeuroSens group in this field is to develop biocompatible nanostructured materials to help the heal of the PNS and cure and study CNS diseases. In particular, at the moment our attention is focused on textured surfaces for helping nerve regeneration and on nanotechnological methods for Globoid Cell Leukodystrophy (or Krabbe disease; OMIM #245200) and Ube3A neurodevelopmental disorders.
Brain-targeted enzyme-loaded nanoparticles: A breach through the blood-brain barrier for enzyme replacement therapy in Krabbe disease
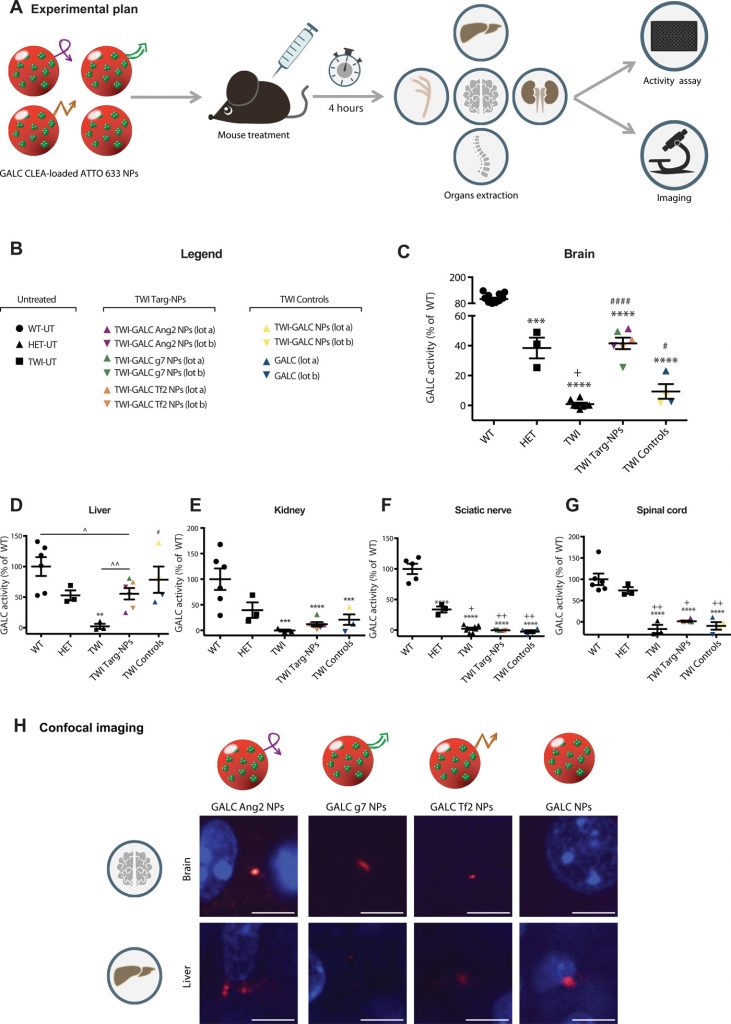
Lysosomal storage disorders (LSDs) result from an enzyme deficiency within lysosomes. The systemic administration of the missing enzyme, however, is not effective in the case of LSDs with central nervous system (CNS)-involvement. Here, an enzyme delivery system based on the encapsulation of cross-linked enzyme aggregates (CLEAs) into poly-(lactide-co-glycolide) (PLGA) nanoparticles (NPs) functionalized with brain targeting peptides (Ang2, g7 or Tf2) is demonstrated for Krabbe disease, a neurodegenerative LSD caused by galactosylceramidase (GALC) deficiency. We first synthesize and characterize Ang2-, g7- and Tf2-targeted GALC CLEA NPs. We study NP cell trafficking and capability to reinstate enzymatic activity in vitro. Then, we successfully test our formulations in the Twitcher mouse. We report enzymatic activity measurements in the nervous system and in accumulation districts upon intraperitoneal injections, demonstrating activity recovery in the brain up to the unaffected mice level. Together, these results open new therapeutic perspectives for all LSDs with major CNS-involvement.
The role of ubiquitin ligase E3A in polarized contact guidance and rescue strategies in UBE3A-deficient hippocampal neurons

