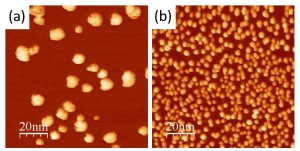
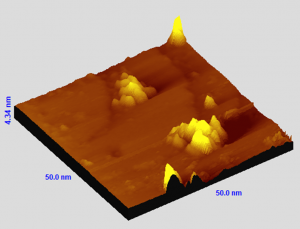
Novel structures of Gallenene intercalated in epitaxial Graphene
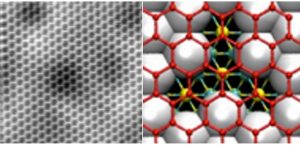
The creation of atomically thin layers of non-exfoliable materials remains a crucial challenge, requiring the development of innovative techniques. Here, confinement epitaxy is exploited to realize two-dimensional gallium via intercalation in epitaxial graphene grown on silicon carbide. Novel superstructures arising from the interaction of gallenene (a monolayer of gallium) with graphene and the silicon carbide substrate are investigated. The coexistence of different gallenene phases, including b010-gallenene and the elusive high-pressure Ga(III) phase, is identified. This work sheds new light on the formation of two-dimensional gallium and provides a platform for investigating the exotic electronic and optical properties of confined gallenene.
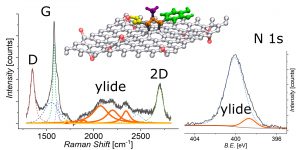
Covalent organic functionalization of graphene via 1,3-dipolar cycloaddition of azomethine ylide
Organic functionalization of graphene is successfully performed via 1,3-dipolar cycloaddition of azomethine ylide in the liquid phase. The comparison between 1-methyl-2-pyrrolidinone and N,Ndimethylformamide as dispersant solvents, and between sonication and homogenization as dispersion techniques, proves N,N-dimethylformamide and homogenization as the most effective choice. The functionalization of graphene nanosheets and reduced graphene oxide is confirmed using different techniques. Among them, energy-dispersive X-ray spectroscopy allows to map the pyrrolidine ring of the azomethine ylide on the surface of functionalized graphene, while micro-Raman spectroscopy detects new features arising from the functionalization, which are described in agreement with the power spectrum obtained from ab initio molecular dynamics simulation. Moreover, X-ray photoemission spectroscopy of functionalized graphene allows the quantitative elemental analysis and the estimation of the surface coverage, showing a higher degree of functionalization for reduced graphene oxide. This more reactive behavior originates from the localization of partial charges on its surface due to the presence of oxygen defects, as shown by the simulation of the electrostatic features. Functionalization of graphene using 1,3-dipolar cycloaddition is shown to be a significant step towards the controlled synthesis of graphene-based complex structures and devices at the nanoscale.
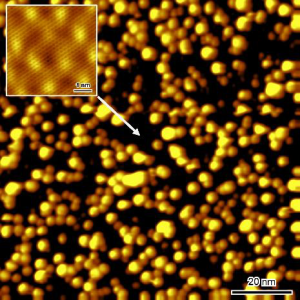
Functionalization of three-dimensional graphene with metal nanoparticles
We demonstrate the first successful functionalization of epitaxial three-dimensional graphene with metal nanoparticles. The functionalization is obtained by immersing the 3D graphene in a nanoparticle colloidal solution. This method is versatile. We demonstrate it with gold and palladium, but it can be extended to other types and shapes of nanoparticles. We have measured the nanoparticle density on the top-surface and in the porous layer volume by Scanning Electron Microscopy and Scanning Transmission Electron Microscopy. Samples exhibit a high coverage of nanoparticles with minimal clustering. We demonstrate that high quality graphene promotes the functionalization, leading to higher nanoparticle densities, both on the surface and in the pores. X-ray Photoelectron Spectroscopy allowed to verify the absence of contamination after the functionalization process. Moreover, it confirmed the thermal stability of the Au- and Pd-functionalized three-dimensional graphene up to 530°C. Our approach opens up new avenues for utilizing three-dimensional graphene as a versatile platform for catalytic applications, sensors, and energy storage and conversion.
Platinum-covered graphene as hydrogen storage medium
The potential of graphene for hydrogen storage, coupled with the established role of Platinum as a catalyst for the hydrogen evolution reaction and the spillover effect, makes Pt-functionalized graphene a promising candidate for near-ambient hydrogen storage. We examine the process of Pt cluster formation on epitaxial graphene and assess the suitability of the system as hydrogen storage material. Scanning tunneling microscopy unveils two primary pathways for Pt cluster growth. In the initial phase, up to 1 ML of Pt coverage, Pt tends to randomly disperse and cover the graphene surface, while the cluster height remains essentially unchanged. Beyond a coverage of 3 ML, the nucleation of new layers on existing clusters becomes predominant. Then, the clusters mainly grow in height. Thermal desorption spectroscopy on hydrogenated Pt-decorated graphene reveals the presence of multiple hydrogen adsorption mechanisms. These measurements demonstrate the ability of Pt-functionalized graphene to store molecular hydrogen at temperatures that are high enough for stable hydrogen binding at room temperature.
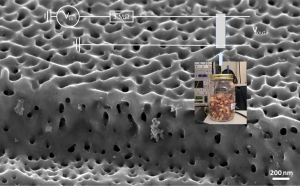
Sensing applications of three-dimensional graphene
Sensors which are sensitive to volatile organic compounds and thus able to monitor the conservation state of food are precious because they work non–destructively and allow to avoid direct contact with the food, ensuring hygienic conditions. In particular, the monitoring of rancidity would solve a widespread issue in food storage. The sensor discussed here is produced utilizing a novel three-dimensional arrangement of graphene, which is grown on a crystalline silicon carbide (SiC) wafer previously porousified by chemical etching. This approach allows a very high surface-to-volume ratio. Furthermore, the structure of the sensor surface features a large amount of edges, dangling bounds, and active sites, which make the sensor, on a chemically robust skeleton, chemically active, particularly to hydrogenated molecules. The interaction of the sensor with such compounds is read out by measuring the sensor resistance in a four wire configuration. The sensor performance has been assessed on three hazelnut samples: sound hazelnuts, spoiled hazelnuts, and stink bug hazelnuts. A resistance variation of about ΔR = 0.13 ± 0.02 Ω between sound and damaged hazelnuts has been detected. Our measurements thus confirm the ability of the sensor to discriminate between sound and damaged hazelnuts. The sensor signal is stable for days, providing the possibility to use this sensor for the monitoring of the storage state of fats and foods in general.
Hydrogen absorption in three-dimensional graphene structures epitaxially grown on 4H SiC(0001)
The use of a novel three-dimensional graphene structure allows circumventing the limitations of the two-dimensional nature of graphene and its application in hydrogen storage. We investigate hydrogen-bonding on monolayer graphene conformally grown via the epitaxial growth method on the (0001) face of a porousified 4H-SiC wafer. Hydrogen absorption is studied via Thermal Desorption Spectroscopy (TDS), exposing the samples to either atomic (D) or molecular (D2) deuterium. The graphene growth temperature, hydrogen exposure temperature, and the morphology of the structure are investigated and related to their effect on hydrogen absorption. The three-dimensional graphene structures chemically bind atomic deuterium when exposed to D2. This is the first report of such an event in unfunctionalized graphene-based materials and implies the presence of a catalytic splitting mechanism. It is further shown that the three-dimensional dendritic structure of the porous material temporarily retains the desorbed molecules and causes delayed emission. The capability of chemisorbing atoms after a catalytic splitting of hydrogen, coupled to its large surface-to-volume ratio, make these structures a promising substrate for hydrogen storage devices.
Hydrogen storage with titanium-functionalized graphene
We report on hydrogen adsorption and desorption on titanium-covered graphene in order to test theoretical proposals to use of graphene functionalized with metal atoms for hydrogen storage. At room temperature, titanium islands grow on graphene with an average diameter of about 10 nm. Samples were then loaded with hydrogen, and its desorption kinetics was studied by thermal desorption spectroscopy. We observe the desorption of hydrogen in the temperature range between 400K and 700 K. Our results demonstrate the stability of hydrogen binding at room temperature and show that hydrogen desorbs at moderate temperatures in line with what is required for practical hydrogen-storage applications.
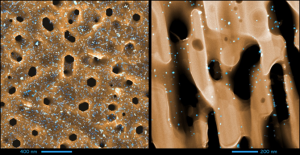
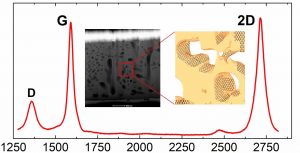
3D arrangement of epitaxial graphene conformally grown on porousified crystalline SiC
Nanoporous materials represent a versatile solution for a number of applications ranging from sensing, energy applications, catalysis, drug delivery, and many others. The synergy between the outstanding properties of graphene with a three-dimensional porous structure, circumventing the limits of its 2D nature, constitutes therefore a breakthrough for many fields. We report the first three-dimensional growth of epitaxial graphene on a porousified crystalline 4H-SiC(0001). The wafer porosification is performed via a sequence of metal-assisted photochemical and photoelectrochemical etching in hydrofluoric acid based electrolytes. Pore dimensions of the matrix have been evaluated by electron tomography resulting in an average diameter of 180 nm. Graphene growth is performed in an ultra high vacuum environment at a base pressure of 10-11 mbar. The graphene growth inside the pores is uniform as confirmed by Transmission Electron Microscopy (TEM) analysis. Raman spectroscopy confirms the high quality of the graphene with a 2D/G intensity ratio > 1 and an average graphene crystal size of 100 nm. Furthermore, it demonstrates a uniform coverage of graphene across the whole sample area. The surface-to-volume ratio of this novel material, its properties, the tunability of the pore size and the scalability of the surface porosification process offer a game changing perspective for a large number of applications.
Morphology and Electronic Properties of Carbonaceous Nanoparticles
Soot nucleation is one of the most complex and debated steps of the soot formation process in combustion. We used scanning tunneling microscopy (STM) and spectroscopy (STS) to probe morphological and electronic properties of incipient soot particles formed right behind the flame front of a lightly sooting laminar premixed flame of ethylene and air. Particles were thermophoretically sampled on an atomically flat gold film on a mica substrate. High-resolution STM images of incipient soot particles were obtained for the first time showing the morphology of sub-5 nm incipient soot particles. High-resolution single-particle spectroscopic properties were measured confirming the semiconductor behavior of incipient soot particles with an electronic band gap ranging from 1.5 to 2 eV, consistent with earlier optical and spectroscopic observations.
An atomically flat single-crystalline gold film thermometer on mica to study energy (heat) exchange at the nano-scale
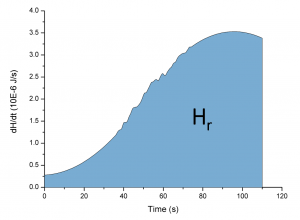
Recently, graphene attracted a lot of attention as an hydrogen storage material owing to its chemical stability, low weight, and favorable physical-chemical properties. In order to enhance the hydrogen adsorption, chemical functionalization of graphene with metal atoms was proposed. In particular, titanium-decorated monolayer graphene (Ti-MLG) has been predicted to allow a gravimetric hydrogen storage density of 7.8 wt%, well above DOE prescriptions. We developed an original experimental setup, in order to measure the enthalpy released during the adsorption process of H2 on a Ti-MLG. The detection of this exothermic release, that follows from the binding energy of H2, is the first direct and accurate measurement of H2 adsorption on functionalized graphene via a calorimetric technique. Because of its non-destructive and simultaneous characteristics, this method introduces a valuable and reliable measurement of the amount of hydrogen stored in the system.
Correlation between morphology and transport properties of quasi-free-standing monolayer graphene
We investigate the morphology of quasi-free-standing monolayer graphene (QFMLG) formed at several temperatures by hydrogen intercalation and discuss its relationship with transport properties. Features corresponding to incomplete hydrogen intercalation at the graphene-substrate interface are observed by scanning tunneling microscopy on QFMLG formed at 600 and 800C. They contribute to carrier scattering as charged impurities. Voids in the SiC substrate and wrinkling of graphene appear at 1000C, and they decrease the carrier mobility significantly.
The Influence of Graphene Curvature on Hydrogen Adsorption: Towards Hydrogen Storage Devices
The ability of atomic hydrogen to chemisorb on graphene makes the latter a promising material for hydrogen storage. On the basis of scanning tunneling microscopy techniques, we report on site-selective adsorption of atomic hydrogen on convexly curved regions of monolayer graphene grown on SiC(0001). This system exhibits an intrinsic curvature owing to the interaction with the substrate. We show that at low coverage hydrogen is found on convex areas of the graphene lattice. No hydrogen is detected on concave regions. These findings are in agreement with theoretical models which suggest that both binding energy and adsorption barrier can be tuned by controlling the local curvature of the graphene lattice. This curvature dependence combined with the known graphene flexibility may be exploited for storage and controlled release of hydrogen at room temperature, making it a valuable candidate for the implementation of hydrogen-storage devices.
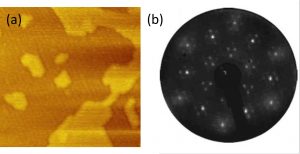
MBE growth of self-assisted InAs nanowires on graphene
Self-assisted growth of InAs nanowires on graphene by molecular beam epitaxy is reported. Nanowires with diameter of ~50 nm and aspect ratio of up to 100 were achieved. The morphological and structural properties of the nanowires were carefully studied by changing the substrate from bilayer graphene through buffer layer to quasi-free-standing monolayer graphene. The positional relation of the InAs NWs with the graphene substrate was determined. A 30° orientation configuration of some of the InAs NWs is shown to be related to the surface corrugation of the graphene substrate. InAs NW-based devices for transport measurements were fabricated, and the conductance measurements showed a semi-ballistic behavior. In Josephson junction measurements in the non-linear regime, Multiple Andreev Reflections were observed, and an inelastic scattering length of about 900 nm was derived.
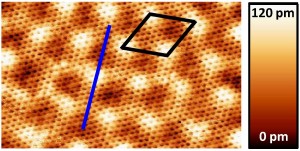
Li-intercalated Graphene on SiC(0001): an STM study
We performed a systematical study via scanning tunneling microscopy (STM) and low-energy electron diffraction (LEED) on the effect of the exposure of Lithium (Li) on graphene on silicon carbide (SiC). We have investigated Li deposition both on epitaxial monolayer graphene and on buffer layer surfaces on the Si-face of SiC. At room temperature, Li immediately intercalates at the interface between the SiC substrate and the buffer layer and transforms the buffer layer into a quasi-free-standing graphene. This conclusion is substantiated by LEED and STM evidence. We show that intercalation occurs through the SiC step sites or graphene defects. We obtain a good quantitative agreement between the number of Li atoms deposited and the number of available Si bonds at the surface of the SiC crystal. Through STM analysis, we are able to determine the interlayer distance induced by Li-intercalation at the interface between the SiC substrate and the buffer layer.
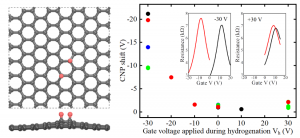
Tuning Hydrogen Adsorption on Graphene by Gate Voltage
To realize applications of hydrogen-adsorbed graphene, a main issue is how to control hydrogen adsorption/desorption at room temperature. We demonstrate the possibility to tune hydrogen adsorption on graphene by applying a gate voltage. The influence of the gate voltage on graphene and its hydrogen adsorption properties was investigated by electrical transport measurements, scanning tunneling microscopy, and density functional theory calculations. We show that more hydrogen adsorbs on graphene with negative gate voltage (p-type doping), compared to that without gate voltage or positive gate voltage (n-type doping). Theoretical calculations explain the gate voltage dependence of hydrogen adsorption as modifications of the adsorption energy and diffusion barrier of hydrogen on graphene by charge doping.
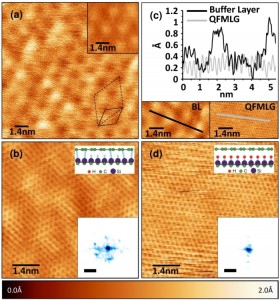
Nanoscale characterisation of graphene and quasi-free standing graphene on SiC(0001)
On the SiC(0001) surface (the silicon face of SiC), epitaxial graphene is obtained by sublimation of Si from the substrate. The graphene film is separated from the bulk by a carbon-rich interface layer (hereafter called the buffer layer) which in part covalently binds to the substrate. Its structural and electronic properties are currently under debate. In the present work we report scanning tunneling microscopy (STM) studies of the buffer layer and of quasi-free-standing monolayer graphene (QFMLG) that is obtained by decoupling the buffer layer from the SiC(0001) substrate by means of hydrogen intercalation. Atomic resolution STM images of the buffer layer reveal that, within the periodic structural corrugation of this interfacial layer, the arrangement of atoms is topologically identical to that of graphene. After hydrogen intercalation, we show that the resulting QFMLG is relieved from the periodic corrugation and presents no detectable defect sites.
Graphene as a medium for hydrogen storage
In the race to develop the next generation of clean fuel, hydrogen is one of the main contenders. One of the main hurdles to overcome before hydrogen can become a widespread energy source in practical applications is the problem of hydrogen storage. In this respect, graphene has recently attracted attention as a promising storage medium. Theoretical studies suggest that it can adsorb up to 9 % mass ratio of hydrogen. However, experimental demonstrations of this have yet to be reported. For this purpose we are using a variable temperature scanning tunneling microscope which operates in ultra high vacuum. The system allows to characterize graphene samples and to study their interaction with hydrogen at the atomic level.